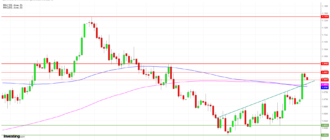
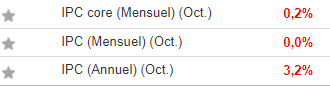
The results of the study of Phase 2b on the Imeglimine conducted in Japan in 299 patients are statistically significant for the primary endpoint and the secondary endpoints
 Download the free guide
Download the free guide
Boost your gains
A significant improvement in liver function was observed. The efficacy and safety in diabetic patients suffering from renal failure are similar to those observed in patients with normal renal function
The results of the study of Phase 2b on the Imeglimine conducted in Japan have been accepted for presentation at the 53rd annual congress of the European Association for the Study of Diabetes (EASD) in September 2017
The Phase 3 program on the Imeglimine Japan is expected to be initiated in the fourth quarter of 2017
The market of diabetes in Japan is growing rapidly and is expected to reach approximately 6 billion$ by 2020*
Lyon, France, on June 6, 2017 8: 00am – POXEL SA (Euronext POXEL – FR0012432516), a biopharmaceutical company specializing in the development of innovative treatments against metabolic diseases, including type 2 diabetes, today announced additional results from the Phase 2b trial on the Imeglimine conducted in Japan. The Company should provide additional data to the first statistically significant results (p