Takeaways
- SpringWorks Therapeutics received FDA approval for the first and only drug to treat desmoid tumors, and shares soared.
- CEO Saqib Islam called the move ” decisive moment. for those suffering from the disease.
- SpringWorks Therapeutics expects the treatment to cost $29,000 for a 30-day supply.
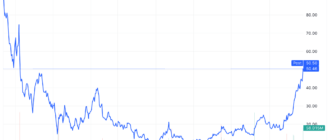
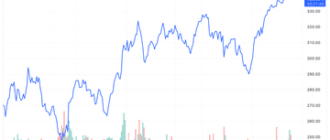
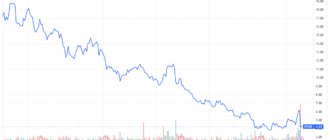
Shares of SpringWorks Therapeutics (SWTX) soared more than 18% on Tuesday after the Food and Drug Administration (FDA) issued its advisory. approval for its pill to be used to treat adults with non-cancerous growths in their connective tissue.
The biotechnology company said the drug, Ogsiveo, is the first and only drug available for patients with progressive desmoid tumors. He added that the FDA's decision was based on a Phase 3 study that showed Ogsiveo met its primary endpoint, “demonstrating a statistically significant improvement over placebo with a reduction in 71% of the risk of disease progression.
CEO Saqib Islam called the move a “watershed moment for the desmoid tumor community” and suggested Ogsiveo “has the potential to become the new standard of care” for those affected. SpringWorks reported that approximately 6,000 people in the United States are being treated for desmoid tumors and more than 20,000 are currently not seeking treatment.
Ogsiveo is the first commercial product for SpringWorks, which was spun off by Pfizer (PFE) in 2017. The company said it would charge $29,000 for a one-month supply.
Even with the Tuesday ahead, SpringWorks Therapeutics stocks remained in negative territory for the year.

TradingView
Do you have a news tip for Investopedia journalists? Please email us at tips@investopedia.com
Source: investopedia.com