Key takeaways
- Sage Therapeutic shares fell to an all-time low after the FDA rejected the use of its treatment for major depressive disorder.
- The drug, made with Biogen, has been approved for to be the very first treatment for postpartum depression.
- The decision led Sage to consider reductions in its pipeline plans and workforce.
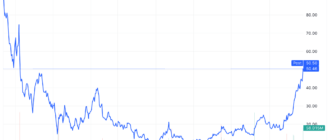
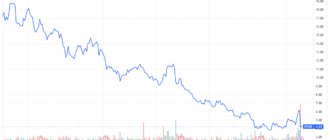
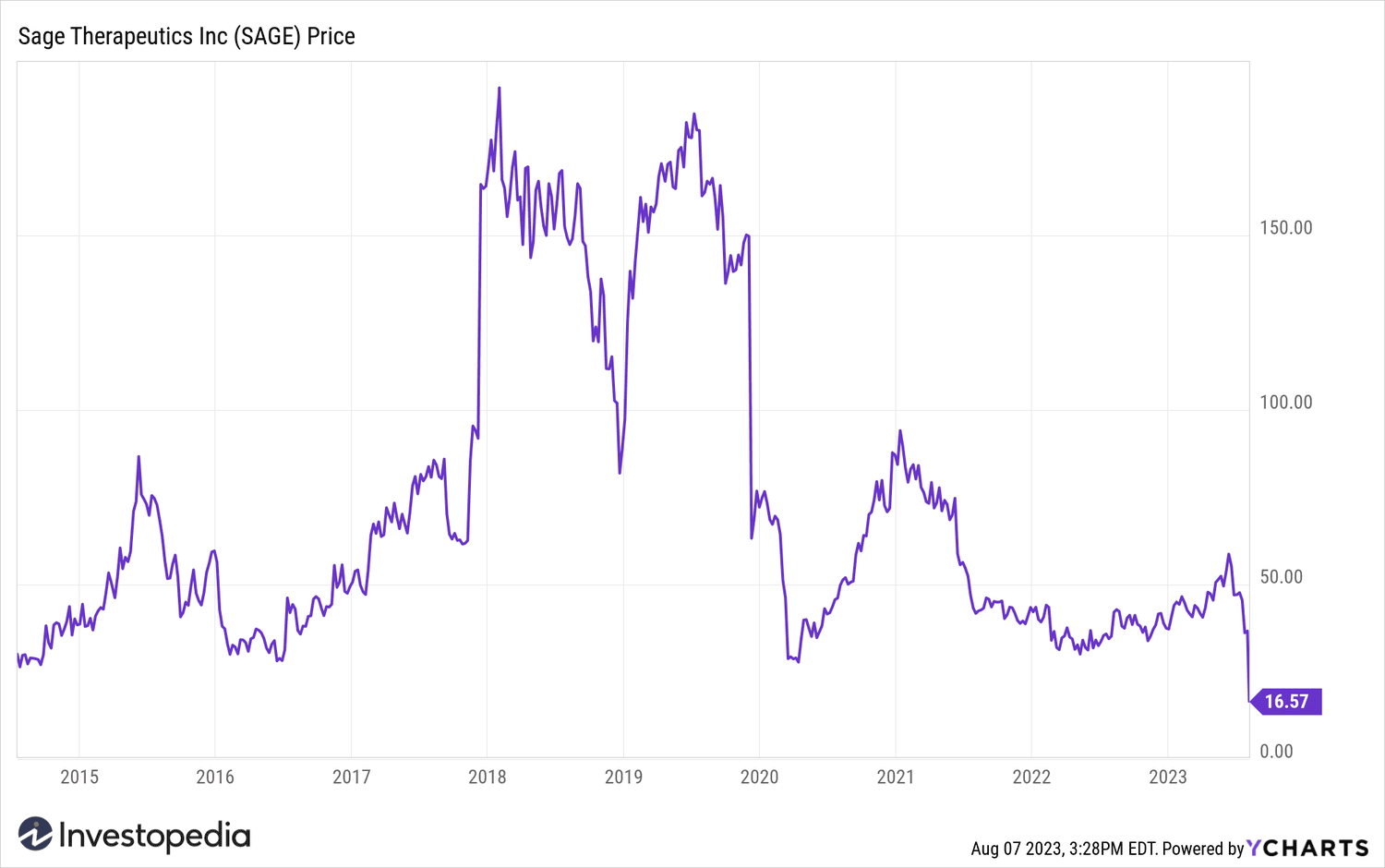
Shares of Sage Therapeutics (SAGE) cratered more than 50% in intraday trading on Monday after the Food and Drug Administration (FDA) ruled that its depression drug, zuranolone, was not effective in the treatment of major depressive disorder (MDD).
The news had initially been positive for Sage and its partner, Biogen (BIIB), on Friday when regulators approved zuranolone to treat women with postpartum depression. This made it the very first drug to gain US approval for this condition. However, the FDA's refusal to expand the use of MDT, also known as clinical depression, removes a much larger potential market for zuranolone.
Barry Greene, CEO of Sage, said “We are very disappointed for patients, especially in the midst of the current mental health crisis and the millions of people with MDD who are struggling to find relief from symptoms.
>
Greene warned that the decision led the company to evaluate resource allocation, “including pipeline prioritization and a reorganization of the workforce with the goal of expanding our cash trail”.
Christopher, CEO of Greene and of Biogen Viehbacher added that the companies “will thoroughly review the FDA's comments on the use of zuranolone in MDD to determine next steps.”
Sage Therapeutics actions have further lost more than half of their value and fell to all-time lows after the news. Biogen shares rose 0.7%.

YCharts
Do you have any news tip for Investopedia reporters? Please email us at tips@investopedia.com
Source: investopedia.com