Key Points
- Regeneron shares soared on indications that the FDA may approve a higher-dose version of its blockbuster eye treatment, Eylea.
- The company anticipates an FDA decision later this quarter.
- Regeneron's earnings and sales beat estimates as sales of its eczema treatment Dupixent soared.
Shares of Regeneron Pharmaceuticals (REGN) surged after the drugmaker reported that he expects the Food and Drug Administration (FDA) may soon approve a higher-dose version of his flagship drug to treat macular degeneration.
The FDA has refused to clear the #39; use of new version of Eylea in June following inspection by third-party manufacturer, Catalent (CTLT). He asked for more information and Regeneron said he expects the paperwork to be submitted to the FDA in the middle of this month. He believes regulators will complete the approval process in the current quarter.
Details on Eylea came in the report on the company's second quarter results, where Regeneron reported earnings of $10.24 per share and revenue of $3.16 billion. Both exceeded analysts' estimates.
Eylea sales fell 7% to $1.5 billion, but also exceeded forecasts. Sales of the eczema drug Dupixent soared 33% to $2.79 billion.
CEO, Dr Leonard Schleifer credited the strong results to Regeneron's “increasingly diverse revenue streams,” adding that the company remains “well positioned for long-term growth.”
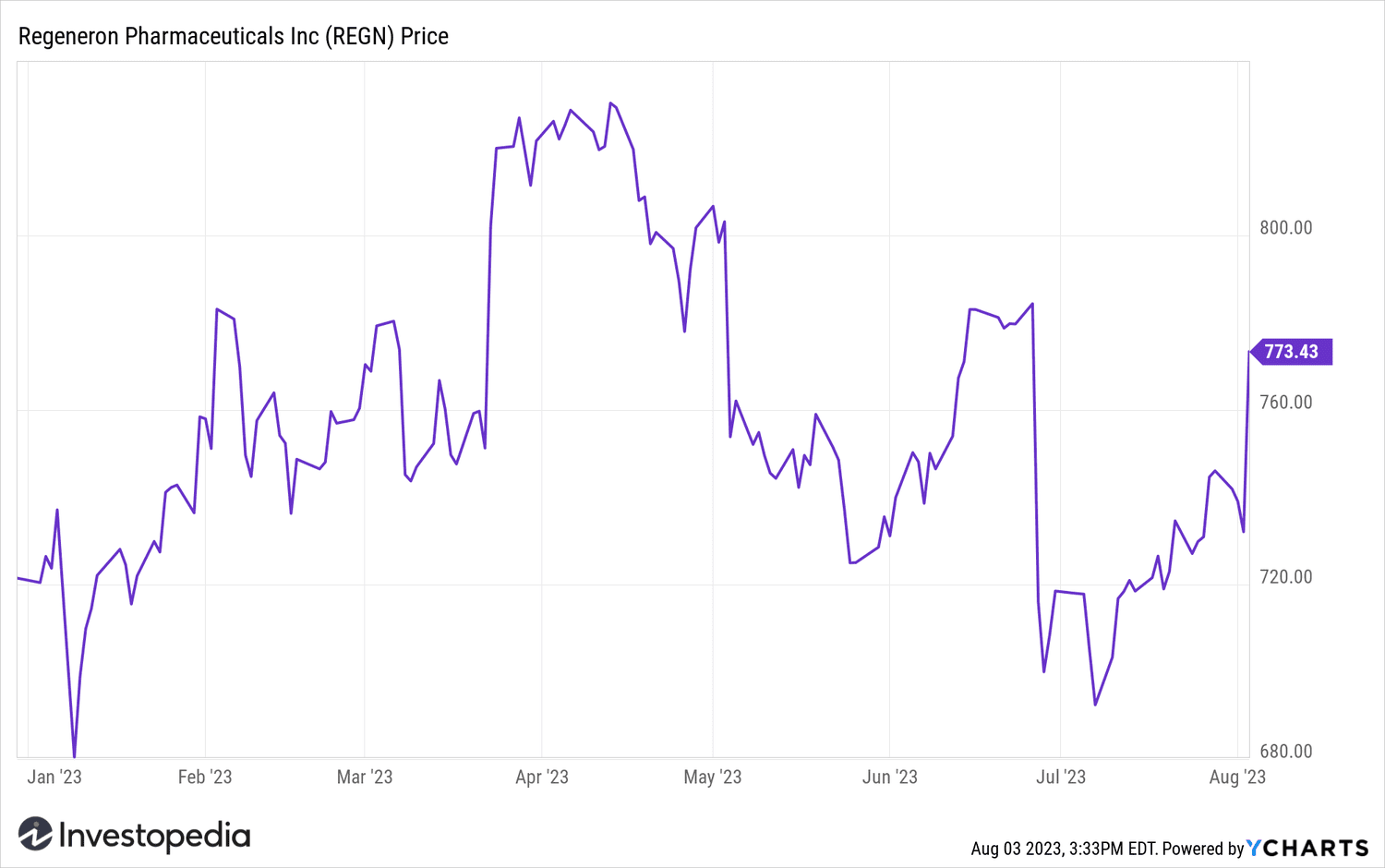
Regeneron Pharmaceuticals shares have rose 5% on Thursday to a more than five-week high.
YCharts
Do you have any news tip for Investopedia reporters? Please email us at tips@investopedia.com
Source: investopedia.com