Takeaways
- The FDA said Philips & #39; management of concerns about its sleep apnea devices has been inadequate.
- The devices were recalled in 2021 because the foam used to reduce noise could break off and be inhaled or swallowed.
- The FDA called on Philips to do more testing, and the company agreed to do so.
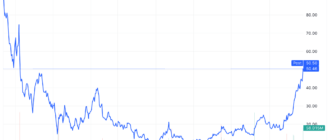
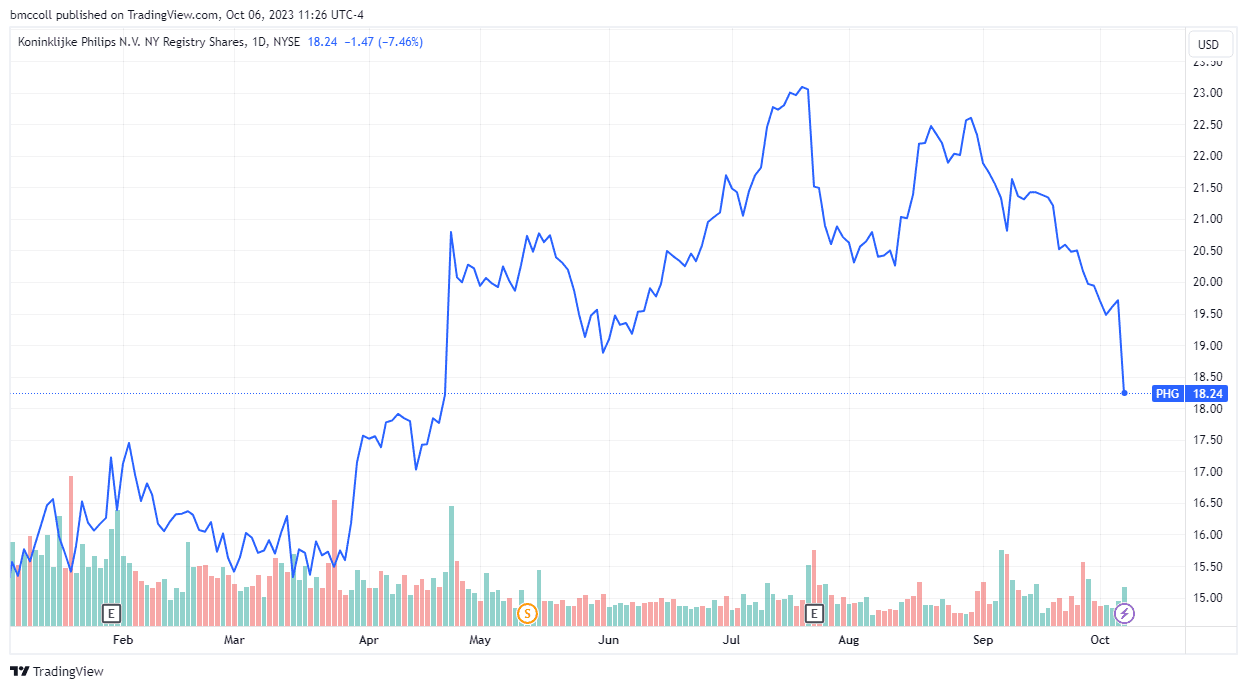
Koninklijke Philips's (PHG) American depositary receipts (ADRs) sank in early trading Friday as the Food and Drug Administration (FDA) ruled that management by the US-based health technology company -Low a sleep apnea device recall was not sufficient.
Philips issued the recall after U.S. regulators warned in June 2021 that millions of Philips ventilators, BiPAP machines and CPAP machines were unsafe because the polyurethane foam they contained contain to reduce noise could decompose and be inhaled or swallowed by people using them.
The FDA indicated that it does not believe that “the tests and analyzes that Philips has shared to date are adequate to fully assess the risks posed to users of the recalled devices. Officials added that additional testing was needed and that Philips had agreed to do so.
The company said it shares the same goal as the FDA and other regulatory agencies, and remains committed to working with them while continuing to “dedicate all necessary resources to ensure that patients receive corrected devices and that the testing and research program is completed.” ."
Philips and its Philips Respironics unit agreed last month in a Pennsylvania court to pay at least $479 million to compensate those who bought the machines.
Koninklijke Philips ADRs were down 7% as of noon ET Friday after the announcement, but were up more than 22% for 2023.
TradingView
Do you have a tip for trading journalists? Investopedia? Please email us at tips@investopedia.com
Source: investopedia.com