Key Points
- Pfizer Shares fell nearly 4% in early trading Monday after development of an oral treatment for obesity and diabetes ended.
- The company said the move was taken after patients showed elevated levels of liver enzymes.
- Pfizer said it would focus on another drug for obesity and diabetes, with Phase 3 trials scheduled for late 2023.
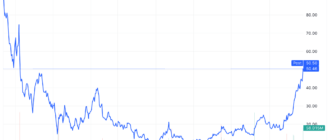
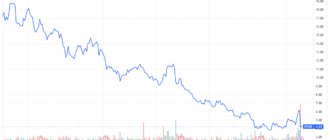
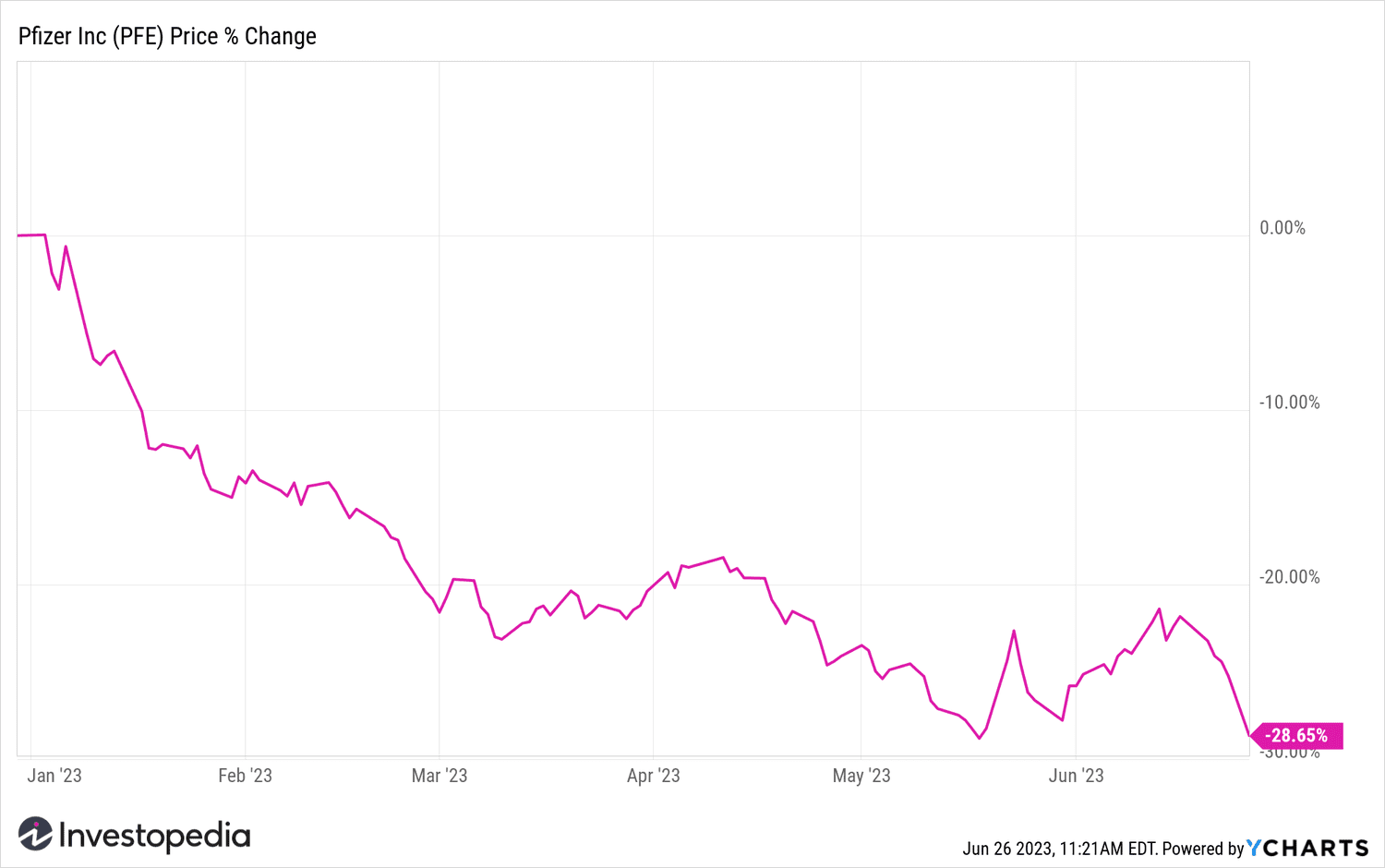
Shares of Pfizer (PFE) fell nearly 4% in early trading Monday after the drugmaker halted development of its experimental obesity and diabetes treatment, lotiglipron, after patients taking the pill recorded high levels of liver enzymes.
The company said it made its decision after the results of a phase 1 study and ongoing phase 2 trials. Pfizer noted that none of the participants experienced liver failure or reported liver-related symptoms or side effects, and no one needed treatment.
Pfizer said it would instead focus its efforts on its other oral obesity and diabetes drug, danuglipron. The company noted that a phase 2 trial showed lower body weight for those who took high-dose versions of the drug twice daily for 16 weeks.
William Sessa, Vice President of Pfizer president and chief scientific officer of internal medicine, indicated that if danuglipron is effective and approved, it could be “in a privileged position” to stand out from similar treatments. Pfizer plans to begin a Phase 3 trial for danuglipron by the end of this year.
Pfizer shares were trading around their year lows after Monday's drop.

YCharts
Do you have any news tip for Investopedia reporters? Please email us at tips@investopedia.com
Source: investopedia.com