Takeaways
- Pfizer ended a study of a twice-daily diet pill due to negative side effects.
- The drug's maker said the treatment was effective, but too many patients stopped taking it after falling ill.
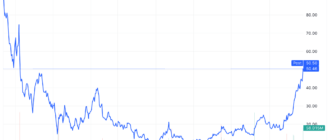
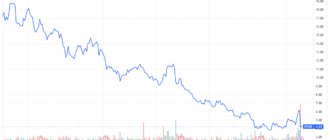
- Pfizer shares fell to their lowest level since March 2020.
Pfizer (PFE) was the worst-performing stock in the S&P 500 on Friday, with shares falling more than 4% in early trading after the drugmaker announced it was ending development of #39;a diet pill to take twice a day due to too many patients. experienced negative side effects.
The company reported that a Phase 2b study of the drug, danuglipron, was effective, “demonstrating a statistically significant change in body weight.”
However, Pfizer added that although the most common side effects were mild and gastrointestinal, half of the people in the study chose to stop taking it, compared to 40% who received a placebo. The company said it would not move forward with a Phase 3 study.
The drug's manufacturer explained that it would now study the effectiveness of a different dosage of danuglipron. Dr. Mikael Dolsten, chief scientific officer and president of Pfizer Research and Development, said the company believes an improved once-daily formulation “could play an important role in the obesity treatment paradigm, and we will focus our efforts on collecting data to understand.” its potential profile.
Pfizer and d' Other pharmaceutical companies are competing to produce an effective oral weight loss treatment. Pfizer's popular Mounjaro and Novo Nordisk's (NVO) Ozempic and Wegovy are injectable products.
The news sent the stocks Pfizer at their lowest level since the start of the COVID-19 pandemic in 2020.


TradingView
Do you have a tip for Investopedia journalists? Please email us at tips@investopedia.com
Source: investopedia.com