Takeaways
- Moderna said its experimental flu vaccine showed positive results in a late-stage trial.
- The company recently received FDA approval for its vaccine against the latest strain of the COVID-19 virus.
- Moderna expects to launch 15 new products over the next five years.
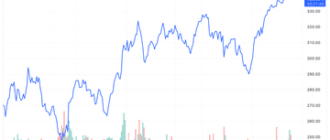
Moderna (MRNA) was one of the best-performing stocks in the S&P 500 as the vaccine maker announced that its experimental flu vaccine met its primary endpoint in a Phase 3 trial.
The treatment, known as mRNA-1010, was effective against all four strains of influenza A and B, the company announced Wednesday. The injection was beneficial for patients of all age groups and, especially, the elderly, the company said.
Moderna also announced that over the next five years it plans to launch 15 new products addressing unmet needs and up to 50 new drugs in clinical trials.
Earlier this week, the U.S. Food and Drug Administration (FDA) approved emergency use authorization for its COVID-19 vaccine that targets the latest strain of the virus. Moderna added that it expects the FDA to authorize its new respiratory syncytial virus (RSV) vaccine for use in April next year.
CEO Stéphane Bancel said that the company's mRNA platform is working and “with significant momentum across the company and our pipeline, we are excited about the near future and focused on execution. »
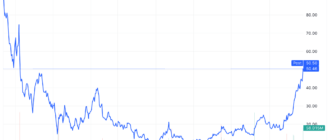
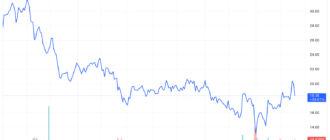
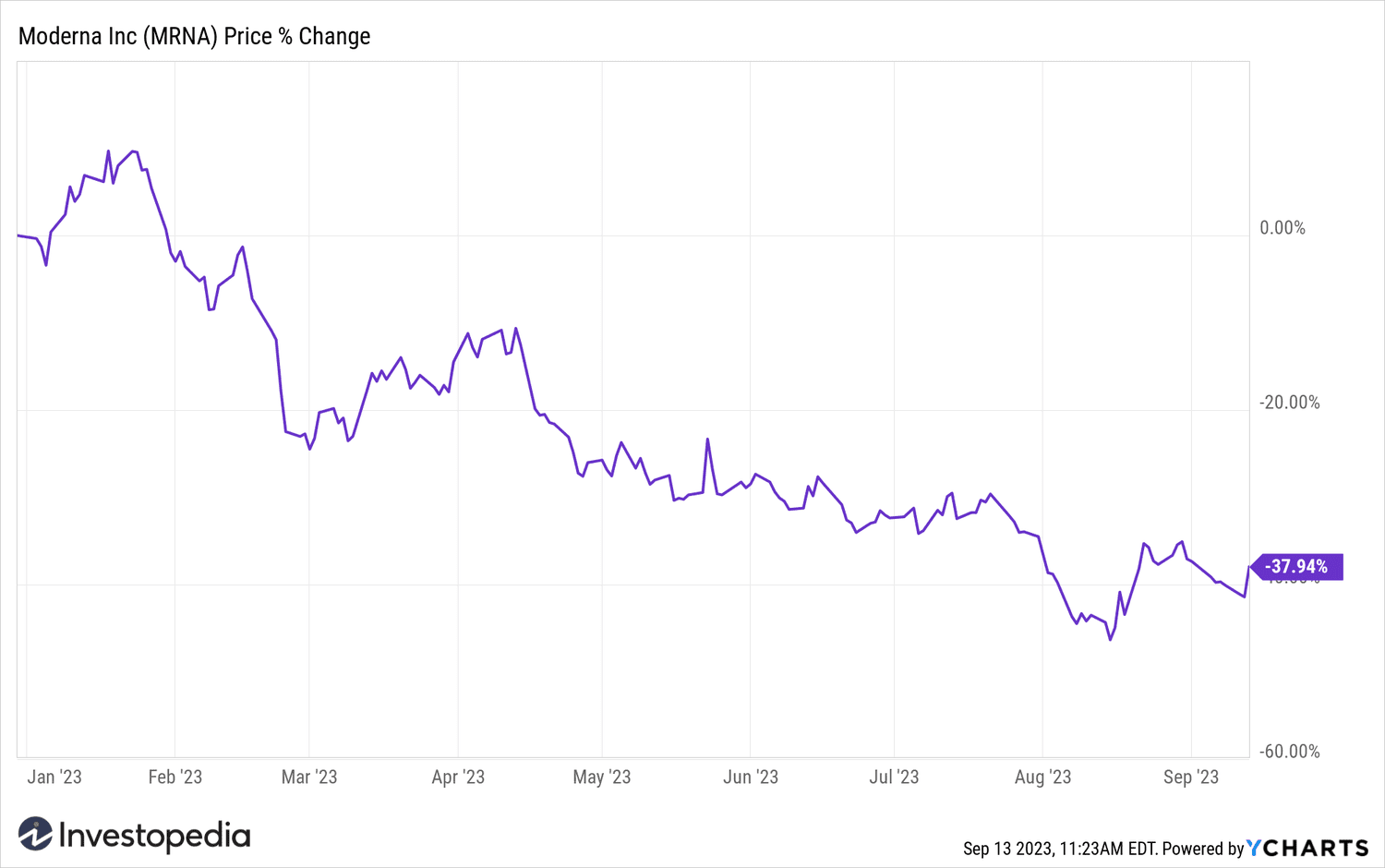
Despite the gains Today, Moderna shares have lost more than a third of their value this year due to falling demand for COVID-19 vaccines.
 Y Charts. Do you have a news tip for Investopedia journalists? Please email us at tips@investopedia.com
Y Charts. Do you have a news tip for Investopedia journalists? Please email us at tips@investopedia.com
Source: investopedia.com