Key Points
- FDA has granted 501(k) clearance to Becton Dickinson's updated BD Alaris Infusion System.
- The system has been subject to multiple recalls, and the CEO Tom Polen said these were resolved in the update.
- Becton Dickinson's shares hit their highest level since 2020 on the news.
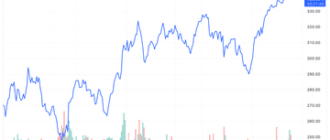
Becton Dickinson (BDX) was the best performing stock in the S&P 500 after Food and Drug Administration (FDA) approval for the medical device maker's updated infusion system.
Becton Dickinson said the FDA has given 501(k) clearance for its BD Alaris Infusion System, which allows hospitals and healthcare systems to run up to four modules for all major types of infusions, including large-volume pumps, syringe pumps, and patient-controlled analgesia (PCA) therapy. with optional respiratory monitoring.
The FDA decision came after BD Alaris faced several recalls. CEO Tom Polen said the new version “addresses all open recalls”, adding that it has “the latest hardware, a new version of software and important cybersecurity updates”.
He explained that the company would now work to bring the updated Alaris BD to customers, initially focusing on fixing or replacing those currently in use in the United States.
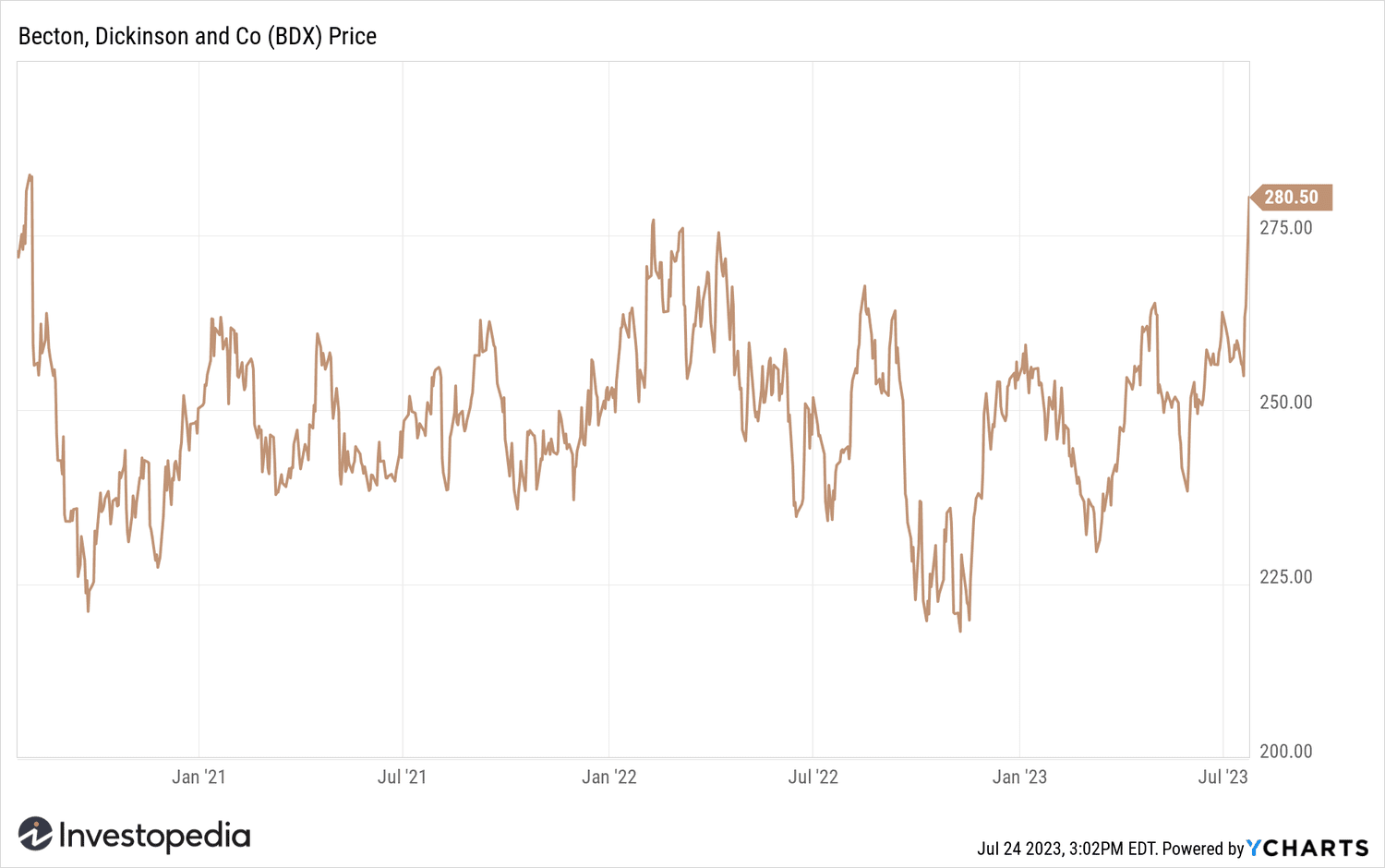
Becton Dickinson shares jumped 5.7% in intraday trading to a three-year high after the news.

YCharts
Do you have any topical advice for Investopedia reporters? Please email us at tips@investopedia.com
Source: investopedia.com